Damascone-Alpha®
Synthétique
Fruity > Berries > Rosy > Minty
Crédits photo: ScenTree SAS
Other names :
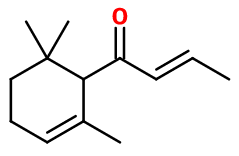
1-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-1-one ; 1-(2,6,6-trimethyl-2-cyclohexen-1-yl)-2-buten-1-one ; Damarose alpha ; Dihydro floriffone A ; 1-(2,6,6-trimethyl-2-cyclohexen-1-yl)-2-butenone
Volatility :
Heart
Uses in perfumery :
Damascone-Alpha® is used in rosy, tuberose, ambery and spicy notes. Gives a green, spicy and fruity facet to floral notes. Very effective, often used diluted.
Natural availability :
Damascone-Alpha® is not available in its natural state.
Year of discovery :
First rose ketones were discovered in 1965, by chemists P. Ruzicka and Dr. Demole, by analyzing Damask Rose Absolute. This opened the way to synthesize a major molecule category of the perfume industry.
Other comments :
Damascone-Alpha® is more powerful and earthy than Damascone-Beta®, which is more fruity and sweet.
Price Range :
€€€
Stability :
Stable in perfumes and diverse functional bases

Crédits photo: ScenTree SAS
- Molecular formula :
- C13H20O
- Molecular Weight :
- 192,3 g/mol
- Density :
- 0,935
- Flash Point :
- >100°C
- Fusion Point :
- 4°C
- Appearance :
- Pale yellow liquid
- Log P :
- 3,66
- Boiling Point :
- 150°C
- Detection Threshold :
- 100 ppb (0,00001%) pour sa forme dextrogyre et 1,5 ppb pour sa forme lévogyre, bien plus puissante,
Synthesis route :
Damascone-Alpha® is part of the ''rose ketones '' family, present in a small amount in Damask Rose Absolute, while playing an important role in their smell. Rose ketones are synthesized from the appropriate derivative of cyclogeranic acid (ester, halide, ...). A reaction of this derivative with an allyl magnesium halide followed by a pyrolysis, allows to obtain the desired compound by rearranging the double bond of the branched chain.
Synthesis precursor :
Damascone-Alpha® is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
In most cases, it is the racemic mixture of the two diastereomers of Damascone-Alpha® that is used in perfumery. Both isomers of the molecule have a rather similar smell, but they can be used separately. The laevorotatory form of Damascone-Alpha® is more powerful than its dextroratatory form.
Ionone alpha and beta are positional isomers of Damascone®, as their ketone function is on another carbon, as well as a methyl function, also relocated. Their smell is therefore very different: the Ionones are reminiscent of violet.
- EINECS number :
- 245-845-8
- FEMA number :
- 3659
- JECFA number :
- 385
- FLAVIS number :
- 07.134
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION
- Amendment :
- 49
- Comments :
- The above limits apply to Rose Ketones used individually or in combination. The sum of concentrations of Rose ketones isomers should not exceed the maximum concentration levels established by this Standard.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,0077 % 0,0023 % 0,046 % 0,043 % 0,011 % 0,011 % 0,011 % 0,011 % 0,025 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,088 % 0,088 % 0,0045 % 0,084 % 0,3 % 0,3 % 0,17 % 0,17 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 44
- Comments :
- The above limits apply to Rose Ketones used individually or in combination. The sum of concentrations of Rose ketones isomers should not exceed the maximum concentration levels established by this Standard.
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,003 % 0,004 % 0,02 % 0,02 % 0,02 % 0,07 % 0,008 % 0,02 % 0,02 % 0,02 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


