Damascone-Delta®
Synthétique
Fruity > Berries > Rosy > Minty > Dry Woods
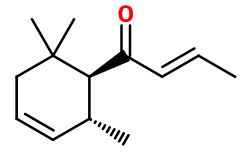
Crédits photo: ScenTree SAS
Other names :
1-(2,6,6-trimethyl-3-cyclohexen-1-yl)-2-buten-1-one ; 8-damascone ; Delta-damascone ; Dihydro floriffone TD ; Delta-rose ketone ; 1-(2,6,6-trimethyl-1-cyclohex-3-enyl)but-2-en-1-one
Volatility :
Heart/Base
Uses in perfumery :
Delta-Damascone® is used for its cooked fruits and jammy sides. It is used most of the time in association with other Damascones to give various effects. It also is frequently used because it is less costly.
Natural availability :
Delta-Damascone® is not found on a natural state. Thus, it is impossible to extract it in any way.
Year of discovery :
First damascones were discovered in 1965 by chemists P. Ruzicka and Dr. Demole, by anayzing an extract as Damask Rose Absolute. This discovery showed the way to a new rosy and fruity molecule family.
Other comments :
The particuarity of Delta-Damascone® is its long-lasting and stability. This ables to use it in Fabric Care products (for clothes).
Price Range :
€€€
Stability :
very stable in perfumes and in various alkaline bases.

Crédits photo: ScenTree SAS
- Molecular formula :
- C13H20O
- Molecular Weight :
- 192,3 g/mol
- Density :
- 0,93
- Flash Point :
- 103°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless to pale yellow liquid
- Log P :
- 4,13
- Boiling Point :
- 264°C
- Detection Threshold :
- 0,007 ng/L air
Synthesis route :
Delta-Damascone® synthesis uses a Diels-Alder reaction (reacting a diene and a dienophile) between 1,3-pentadiene and mesityl oxide, giving birth to acetyl trimethyl cyclohexene. This intermediary product can then handle an aldol condensation with Acetaldehyde, to obtain the final product : Delta-Damascone®.
Synthesis precursor :
Delta-Damascone® is not a precursor for the synthesis of another olfactive compound.
Isomerism :
''Rose ketone '' family contains a few different molecules, almost all evoking cooked apple, blackcurrant and Damask Rose Absolute. Delta-Damascone® smell is close to Alpha-Damascone®, which is a little more woody. Beta-Damascone® and Gamma-Damascone® have respectively more rosy and coniferous smells.
Alpha-Ionone and Beta-Ionone are both positional isomers of Damascone® molecules, because their ketone function is located on another carbon, and as they have a relocated methyl function. Nevertheless, their smell is very different, as Ionones are reminiscent of violet flower.
- EINECS number :
- 260-709-8
- FEMA number :
- 3622
- JECFA number :
- 386
- FLAVIS number :
- 07.130
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION
- Amendment :
- 49
- Comments :
- The above limits apply to Rose Ketones used individually or in combination. The sum of concentrations of Rose ketones isomers should not exceed the maximum concentration levels established by this Standard.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,0077 % 0,0023 % 0,046 % 0,043 % 0,011 % 0,011 % 0,011 % 0,011 % 0,025 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,088 % 0,088 % 0,0045 % 0,084 % 0,3 % 0,3 % 0,17 % 0,17 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- Amendment :
- 44
- Comments :
- The above limits apply to Rose Ketones used individually or in combination. The sum of concentrations of Rose ketones isomers should not exceed the maximum concentration levels established by this Standard.
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,003 % 0,004 % 0,02 % 0,02 % 0,02 % 0,07 % 0,008 % 0,02 % 0,02 % 0,02 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

