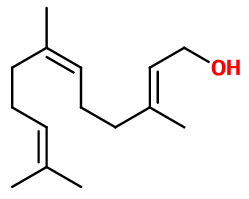
Farnesol
Naturelle - Synthétique
Floral > Light Flowers > Green Fruits > Green
Crédits photo: ScenTree SAS
Other names :
Farnesyl alcohol ; 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol ; Dragosantol
Volatility :
Base
Uses in perfumery :
Farnesol is used for lily of the valley notes, to bring a fruity nuance. It is also used as a fixative.
Natural availability :
Farnesol can be extracted from Ambrette Seeds Absolute, because it is found there in its (Z,E) conformation. It is also found in Petitgrain Bigarade EO and Orange Blossom Absolute, from which it can be extracted.
Year of discovery :
Discovered in 1913
Other comments :
The smell of Farnesol becomes stronger when the molecule is evaporating, surely due to oxydation.
Farnesol is part of the sesquiterpene molecule category. This means that this molecule has fifteen carbon atoms, that is three isoprene units. Terpenes (monoterpenes, diterpenes, sesquiterpenes...) were discovered thanks to the Diels-Alder reaction, involving a diene and a dienophile, abling to modelize terpenes and to obtain a great variety of them.
Farnesol is part of the sesquiterpene molecule category. This means that this molecule has fifteen carbon atoms, that is three isoprene units. Terpenes (monoterpenes, diterpenes, sesquiterpenes...) were discovered thanks to the Diels-Alder reaction, involving a diene and a dienophile, abling to modelize terpenes and to obtain a great variety of them.
Price Range :
€€€
Stability :
Terpenes may polymerize under strong oxydation.

Crédits photo: ScenTree SAS
- Molecular formula :
- C15H26O
- Molecular Weight :
- 222,37 g/mol
- Density :
- 0,88
- Flash Point :
- 96°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- 5,7
- Boiling Point :
- 107°C
- Detection Threshold :
- De l'ordre de 1 ppm (0,0001%)
Synthesis route :
Synthetic Farnesol can be obtained by isomerization of Nerolidol. This synthesis is mostly replacing the natural extraction of Farnesol, as it was formerly usual.
Synthesis precursor :
Farnesol is not used for the synthesis of another compound of olfactive interest.
Isomerism :
Farnesol has four stereoisomers, due to the presence of two double bonds. Thus, these two bonds can have either a cis (Z) or trans (E) configuration. The four stereoisomer couples of Farnesol are : (Z,Z), (E,E), (Z,E) and (E,Z). In perfumery, a mixture of isomers is used most of the time, because no real olfactive difference exist between the isomers.
- EINECS number :
- 225-004-1
- FEMA number :
- 2478
- JECFA number :
- 1230
- FLAVIS number :
- 02.029
- Allergens :
- Farnesol may provoke an allergic reaction on skin contact (redness, heat, scraching, prickling) for some people.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION_SPECIFICATION
- Cause of restriction :
- DERMAL SENSITIZATION
- Amendment :
- 49
- Comments :
- Farnesol should only be used as a fragrance ingredient if it contains a minimum of 96% of farnesol isomers as determined by GLC.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,21 % 0,062 % 1,2 % 1,2 % 0,29 % 0,29 % 0,29 % 0,29 % 0,68 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 2,4 % 2,4 % 0,12 % 2,3 % 8,1 % 8,1 % 4,5 % 4,5 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- Amendment :
- 40
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,08 % 0,11 % 0,4 % 1,2 % 0,6 % 2 % 0,2 % 2 % 5 % 2,5 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


