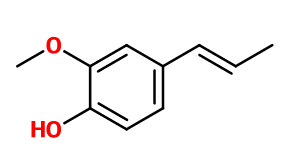
Isoeugenol
Naturelle - Synthétique
Spicy > Warm Spices > Eugenolic > Yellow Fruits
Crédits photo: ScenTree SAS
Other names :
2-methoxy-4-prop-1-en-2-ylphenol ; 4-hydroxy-3-methoxy-1-propen-1-yl benzene ; 4- hydroxy-3-methoxy-1-propenyl benzene ; 4- hydroxy-3-methoxypropenyl benzene ; 2-methoxy-4-(1-methylethenyl)phenol ; 2-methoxy-4-(1-methylvinyl)phenol ; 2-methoxy-4-(1-propenyl)phenol ; 3-methoxy-4-hydroxy-1-propen-1-yl benzene ; 2-methoxy-4-propenyl phenol ; 4-propenyl guaiacol
Volatility :
Heart
Uses in perfumery :
Isoeugenol is used in carnation, spicy and ambery notes, often combined with Eugenol. Often replaced by Acetyl-Isoeugenol, unregulated.
Natural availability :
In many cases, naturally present Isoeugenol is accompanied by Eugenol, which is present in greater quantities in the following raw materials. Isoeugenol can be extracted from Clove Bud EO (1%), betel leaf (about 10%) or Ylang-Ylang Extra EO, or other fractions (up to 0.5% depending on the fractions).
Year of discovery :
Data not available.
Other comments :
Isoeugenol is one of the 26 allergens in perfumery.
Smokier and more rounded than Eugenol, but less floral and metallic. Slightly more expensive than Eugenol.
Smokier and more rounded than Eugenol, but less floral and metallic. Slightly more expensive than Eugenol.
Price Range :
€€
Stability :
Becomes red under the effect of light. This raw material is not convenient in every functional base : can't be used in a candle or shower gel base.

Crédits photo: ScenTree SAS
- Molecular formula :
- C10H12O2
- Molecular Weight :
- 164,2 g/mol
- Density :
- 1,087
- Flash Point :
- 112°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Pinkish liquid
- Log P :
- 2,65
- Boiling Point :
- 270°C
- Detection Threshold :
- 100 ppb (0,00001%),
Synthesis route :
Isoeugenol can be produced from Eugenol. Forming sodium or potassium salts from Eugenol allows to isomerize the molecule by heating it in order to obtain Isoeugenol by an acid treatment. This isomerization can also be obtained directly by a ruthenium or rhodium catalysis.
Synthesis precursor :
Isoeugenol is a precursor to the synthesis of several compounds of olfactory interest. Catalytic hydrogenation of the molecule allows to obtain Dihydoeugenol. In the past, Vanillin was produced by an oxidation reaction on Isoeugenol. The alcohol function of the molecule can finally be used for several esterification reactions, to obtain Isoeugenyl acetate for example.
Isomerism :
Isoeugenol has two diastereomers with a similar smell. It is the mixture of these two isomers that is the most used in perfumery. The trans isomer is nevertheless the most present in this mixture, as it is thermodynamically more stable. Eugenol is an isomer of Isoeugenol, as the double bond present in the two molecules is simply relocated from one molecule to another. Its smell is less fruity but more woody and vanillic.
Styrallyl acetate and Frambinone® are examples of constitutional isomers of Isoeugenol. Their smell is however very different.
- EINECS number :
- 202-590-7
- FEMA number :
- 2468
- JECFA number :
- 1260
- FLAVIS number :
- 04.004
- Allergens :
- Isoeugenol may provoke an allergic reaction on skin contact (redness, heat, scraching, prickling) for some people.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,019 % 0,0057 % 0,12 % 0,11 % 0,027 % 0,027 % 0,027 % 0,009 % 0,063 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,22 % 0,22 % 0,009 % 0,21 % 0,21 % 0,75 % 0,009 % 0,009 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 43
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,01 % 0,01 % 0,02 % 0,02 % 0,02 % 0,2 % 0,02 % 0,02 % 0,02 % 0,02 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.



