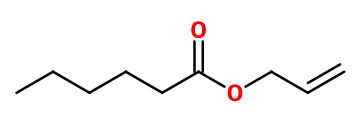
Allyl Caproate
Naturelle - Synthétique
Fruity > Tropical Fruits > Butyric
Crédits photo: ScenTree SAS
Other names :
Allyl Hexanoate ; Prop-2-en-1-yl hexanoate ; Prop-2-en-1-yl caproate ; Prop-2-enyl hexanoate ; Prop-2-enyl caproate ; 2-propenyl hexanoate ; 2-propenyl caproate
Volatility :
Head
Uses in perfumery :
Allyl Caproate is used in pineapple and apple notes, for a ripe and sweet effect. Also used in exotic and yellow fruit notes.
Natural availability :
Allyl Caproate is present in pineapple and mushroom. There is also an Allyl Caproate of natural origin.
Year of discovery :
Data not available.
Other comments :
Data not available.
Price Range :
€
Stability :
May form caproic acid through time

Crédits photo: ScenTree SAS
- Molecular formula :
- C9H16O2
- Molecular Weight :
- 156,23 g/mol
- Density :
- 0,889
- Flash Point :
- 74°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- 3,2
- Boiling Point :
- 190°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Allyl Caproate is prepared by esterification between Hexanoic acid and allylic alcohol (or prop-2-en-1-ol), by acid catalysis.
Synthesis precursor :
Allyl Caproate is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
Isoamyl Butyrate and gamma-Nonalactone are constitutional isomers of Allyl Caproate. Isoamyl Butyrate also has a fruity and butyric smell, but gamma-Nonalactone has a very different note of peach and coconut.
- EINECS number :
- 204-642-4
- FEMA number :
- 2032
- JECFA number :
- 3
- FLAVIS number :
- 09.244
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is not restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

