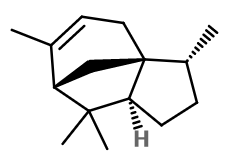
Alpha-Cedrene
Naturelle - Synthétique
Woody > Cedar > Smoky Woods > Leather
Crédits photo: ScenTree SAS
Other names :
(3R-(3a,3ab,7b,8aa))-2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulene ; (1S,2R,5S)-2,6,6,8- tetramethyl tricyclo(5.3.1.01.5)undec-8-ene
Volatility :
Base
Uses in perfumery :
Alpha-Cedrene is generally used for leather notes or as a substitute for Cedarwood Virginia EO, for its substantially equivalent note.
Natural availability :
Alpha-Cedrene is one of the major components of Cedarwood Virginia EO, Cedarwood Texas EO, Cedarwood Chinese EO, among others, and is present in trace amounts especially in Verbena EO.
Year of discovery :
Isolated by scientist Walter in 1841 (ex. Juniperus virginiana).
In 1953, Stork and Breslow were the first to synthesize dl-cedrol and dl-Alpha-Cedrene. Two years later, they acheived in synthesizing completely cedrol and Alpha-Cedrene.
Other comments :
Alpha-Cedrene is a sesquiterpene. This means that it has 15 carbon atoms, linked to 24 hydrogen atoms. In the case of Alpha-Cedrene, the molecule has a tricyclic structure.
Price Range :
€€
Stability :
Terpenes tend to polymerize by oxydation

Crédits photo: ScenTree SAS
- Molecular formula :
- C15H24
- Molecular Weight :
- 204,35 g/mol
- Density :
- 0,93
- Flash Point :
- 104°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- Donnée indisponible,
- Boiling Point :
- 262°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
There are several routes of synthesis for Alpha-Cedrene depending on the starting reagent. Only one of them is detailed below:
One of the syntheses with the least steps is a Corey synthesis from 2-methylhept-2-enen lithiated in its carbon 6, reacting with 3-methoxycyclopent-2-enone. The obtained intermediate product undergoes a reduction with sodium borohydride, then a treatment with diiodomethane, catalysed by brass, and a pyrogenation in the presence of chromium trioxide and dichloromethane. An acetylation of the ketone function that is still present, allows to form a carbocation which, by rearrangement, forms epi-Alpha-Cedrene and Alpha-Cedrene.
Synthesis precursor :
Alpha-Cedrene is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
Alpha-Cedrene has several isomers, by delocalization of its double bond. We distinguish beta-Cedrene, which has a smell relatively similar to Alpha-Cedrene.
Moreover, this molecule is a constitutional isomer of beta-Caryophyllene and Valencene, but has a smell much less spicy or green than these two isomers.
- EINECS number :
- 207-418-4
- FEMA number :
- Donnée indisponible.
- JECFA number :
- Donnée indisponible.
- FLAVIS number :
- 01.022
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION
- Amendment :
- 49
- Comments :
- The natural contribution of Cedrene is determined by the sum of the natural contributions of each of its isomers.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,27 % 0,08 % 1,6 % 1,5 % 0,38 % 0,38 % 0,38 % 0,38 % 0,88 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 3,1 % 3,1 % 0,16 % 2,9 % 11 % 11 % 5,8 % 5,8 % No Restriction
This ingredient is not restricted for the 48th amendment
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.co to learn about our advertising opportunities.
