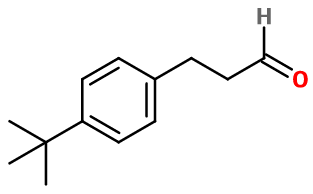
Bourgeonal
Synthétique
Floral > Light Flowers > Aldehydic > Aquatic > Green
Crédits photo: ScenTree SAS
Other names :
Burgenal ; 3-(4-tert-butylphenyl)propanal ; 4-(1,1-dimethylethyl)-benzenepropanal ; Para-tertbutyl dihydrocinnamaldehyde ; Para-tertbutyl dihydrocinnamic aldehyde ; 3-(4-tert-butylphenyl)propionaldehyde ; 4-(1,1-dimethyl ethyl) benzene propanal ; Langeonal ; Lilional ; Liliphenal ; 3-[4-(2-methyl-2-propanyl)phenyl]propanal
Volatility :
Heart
Uses in perfumery :
Bourgeonal is used in all types of perfumery, in floral-aldehydic, green notes. Often used for its stability in soap and detergent bases, in addition to alcoholic perfumery.
Natural availability :
Bourgeonal is not available in its natural state.
Year of discovery :
1959
Other comments :
Compared to Lilial® or Silvial®, Bourgeonal is a floral-aldehydic molecule also having a green note, making the difference with other molecules of this type.
Price Range :
€€
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Exclusively stable in fabric conditioners, shampoos and hair conditioners.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Exclusively stable in fabric conditioners, shampoos and hair conditioners.

Crédits photo: ScenTree SAS
- Molecular formula :
- C13H18O
- Molecular Weight :
- 190,28 g/mol
- Density :
- 0,961
- Flash Point :
- 73°C
- Fusion Point :
- -11°C
- Appearance :
- Colorless liquid
- Log P :
- 3,2
- Boiling Point :
- 207°C
- Detection Threshold :
- 0,4 ng/L air
Synthesis route :
Bourgeonal can be synthesized from 4-tert-Butyl benzaldehyde by an aldol reaction with Acetaldehyde. The intermediate product that is obtained is 4-tert-Butyl Cinnamaldehyde. A catalytic hydrogenation converts this intermediate into Bourgeonal. Another synthetic route reacts 4-tert-Butyl benzene with acrolein diacetate in the presence of a Lewis acid.
Then, the intermediate product is subjected to a saponification.
Synthesis precursor :
Bourgeonal forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Isomerism :
The meta and ortho isomers of Bourgeonal are not used in perfumery.
Cyclamen Aldehyde is a positional isomer of Bourgeonal. However, its smell is quite different, as it is more marine. Both have an aldehydic and floral-white flowers smell.
- EINECS number :
- 242-016-2
- FEMA number :
- Donnée indisponible.
- JECFA number :
- Donnée indisponible.
- FLAVIS number :
- Donnée indisponible.
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,0041 % 0,025 % 0,025 % 0,47 % 0,12 % 0,029 % 0,037 % 0,0096 % 0,087 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,029 % 0,029 % 0,0096 % 0,099 % 0,099 % 0,24 % 0,0096 % 0,0096 % 6,9 % - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 43
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,03 % 0,04 % 0,2 % 0,5 % 0,3 % 0,8 % 0,1 % 0,6 % 0,6 % 0,6 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

