Fraistone®
Synthétique
Fruity > Green Fruits > Anisic > Green
Crédits photo: ScenTree SAS
Other names :
Fragolane® ; Dimethyl Dioxolan ; Fraisberry® ; Ethyl 2-(2,4-dimethyl-1,3-dioxolan-2-yl) acetate ; 2,4-dimethyl-1,3-dioxolane-2-ethyl acetate ; Dimethyldioxolan ; Ethyl 2-(2,4-dimethyl-1,3-dioxolan-2-yl)acetate ; Ethyl aceto acetate PG acetal ; Ethyl acetoacetate propylene glycol ketal ; Fructone B ; Fruity ketal ; Propyl fruitat ; Strawberry ketal
Volatility :
Head/Heart
Uses in perfumery :
Fraistone® is used in fruity and floral notes of fruity rose, tuberose, jasmine, orange blossom and syringua.
Natural availability :
Fraistone® is not available in its natural state.
Year of discovery :
1937
Other comments :
Data not available.
Price Range :
€€
Stability :
Stable in perfumes and diverse functional bases

Crédits photo: ScenTree SAS
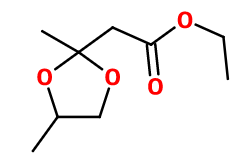
- Molecular formula :
- C9H16O4
- Molecular Weight :
- 188,22 g/mol
- Density :
- 1,042
- Flash Point :
- 91°C
- Fusion Point :
- -68°C
- Appearance :
- Colorless liquid
- Log P :
- 1,5
- Boiling Point :
- 85°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Fraistone® is an acetal of Ethyl Acetoacetate (a synthesis based on formic acid and acetone in its enolic form). It is obtained by reaction between this reagent and propan-1,2-diol.
Synthesis precursor :
Fraistone® is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
Fraistone® has two asymmetric carbons. It is however a mixture of isomers that is used in perfumery.
- EINECS number :
- 228-536-2
- FEMA number :
- 4294
- JECFA number :
- 1715
- FLAVIS number :
- 06.087
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is not restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

