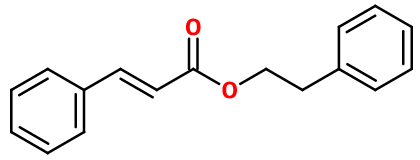
Phenyl Ethyl Cinnamate
Naturelle - Synthétique
Floral > Rosy > Cinnamic
Crédits photo: ScenTree SAS
Other names :
Benzyl carbinyl 3-phenylpropenoate ; Benzyl carbinyl cinnamate ; Beta-phenylethyl-beta-phenylacrylate ; 2-phenylethyl 3-phenylpropenoate ; Phenylethyl cinnamate ; 3-phenyl-2-propenoate de phenylethyl ; 3-phenylprop-2-enoate de phenylethyl
Volatility :
Base
Uses in perfumery :
Phenyl Ethyl Cinnamate is used to bring volume and as a fixative in perfumes. It enlarges the perfume and brings tenacity, especially for rosy notes.
Natural availability :
Phenyl Ethyl Cinnamate can be extracted from Populus balsamifera bud extract. Nevertheless, the synthetic molecule is used most of the time in perfumery.
Year of discovery :
Data not available.
Other comments :
Phenyl Ethyl Cinnamate is almost odorless. It can only be used for its fixative effect, rather than its particular smell.
Its solubility in alcool is very week. Dipropylene Glycol is usually necessary to solubilize this molecule.
Its smell is less medicinal than the one of Ethyl Cinnamate.
Its solubility in alcool is very week. Dipropylene Glycol is usually necessary to solubilize this molecule.
Its smell is less medicinal than the one of Ethyl Cinnamate.
Price Range :
€€
Stability :
Esters may form their corresponding acid in stability

Crédits photo: ScenTree SAS
- Molecular formula :
- C17H16O2
- Molecular Weight :
- 252,31 g/mol
- Density :
- Donnée indisponible.
- Flash Point :
- 113°C
- Fusion Point :
- 54°C
- Appearance :
- White crystals
- Log P :
- 4,6
- Boiling Point :
- 195°C (à 2,6 mbar)
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Phenyl Ethyl Cinnamate is synthesized by an esterification reaction involving cinnamic acid and Phenyl Ethyl Alcohol. This reaction uses a small quantity of catalysor as concentrated sulfuric acid. A better synthesis yield can result from this reaction by using chlorocinnamic acid or cinnamic anhydride, more costly. This synthesis involves the use of the trans isomer of cinnamic acid.
Synthesis precursor :
Phenyl Ethyl Cinnamate is not a precursor for the synthesis of another compound of olfactive interest.
Isomerism :
Phenyl Ethyl Cinnamate used in perfumery corresponds to the trans isomer of this molecule. The cis isomer has a relatively similar smell.
- EINECS number :
- 203-120-3
- FEMA number :
- 2863
- JECFA number :
- 671
- FLAVIS number :
- 09.743
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is not restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

