Polysantol®
Synthétique
Woody > Sandalwood > Milky > Buttery
Crédits photo: ScenTree SAS
Other names :
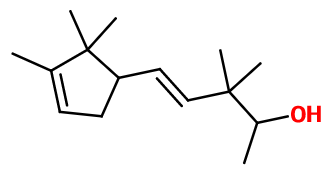
Mysantol® ; (E)-3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-penten-2-ol ; Aquisantol ; Lansantol ; Megasantol ; Nirvanol ; Santol pentenol ; Supersantol ; Trimethyl cyclopentenyl dimethyl isopentenol
Volatility :
Base
Uses in perfumery :
Polysantol® is used in woody accords, linked with musky notes, in milk accords and in sandalwood notes in woody perfumes. Brings a cosmetic note.
Natural availability :
Polysantol® is not available in its natural state.
Year of discovery :
1984
'Polysantol®' trademark has been published and protected by Firmenich SA since 22/10/1985 (brand N°497404)
Other comments :
Several sandalwood compounds have similar structures. This is the case of Ebanol, Bacdanol®, Javanol® and Polysantol®. Their structure is composed of a branched cyclopentenic body, and a long carbon chain attached to this cycle. It is on this chain that the differences between the compounds are made.
Price Range :
€€
Stability :
Stable in perfumes and diverse functional bases

Crédits photo: ScenTree SAS
- Molecular formula :
- C15H26O
- Molecular Weight :
- 222,37 g/mol
- Density :
- 0,902
- Flash Point :
- >100°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- 4,7
- Boiling Point :
- Donnée indisponible.
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
Polysantol® is synthesized from campholenaldehyde, condensed with butan-2-one in an acid medium to give 3-methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-3-penten-2-one as an intermediate product. A methylation step under phase transfer conditions provides a new product, which can be reduced by sodium borohydride to get Polysantol®.
Synthesis precursor :
Polysantol® is not used to synthesize other compounds of organoleptic interest.
Isomerism :
Polysantol® has a double bond and two asymmetric carbons that give rise to several isomers of this molecule. It is nevertheless its mixture of isomers that is used in perfumery.
In addition, Polysantol® is a constitutional isomer of Javanol®. These two compounds have a similar structure, and are used for similar reasons in perfumery. Javanol® has a less milky note than Polysantol®, but is more powerful.
- EINECS number :
- 411-580-3
- FEMA number :
- Donnée indisponible.
- JECFA number :
- Donnée indisponible.
- FLAVIS number :
- Donnée indisponible.
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,031 % 0,057 % 0,25 % 1,1 % 0,27 % 0,27 % 0,27 % 0,091 % 0,031 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,63 % 0,63 % 0,091 % 1,7 % 1,7 % 4 % 0,091 % 0,091 % No Restriction
This ingredient is not restricted for the 48th amendment
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


