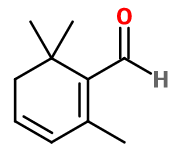
Safranal
Synthétique
Spicy > Warm Spices > Saffron
Crédits photo: ScenTree SAS
Other names :
2,6,6-trimethylcyclohexa-1,3-diene-1-carbaldehyde ; Dehydro beta-cyclocitral ; 2,3-dihydro-2,2,6-trimethyl benzaldehyde ; Safralan ; Safranal P ; 2,6,6-trimethyl cyclohexa-1,3-dienyl methanal
Volatility :
Head/Heart
Uses in perfumery :
Safranal is used in fruity notes for its characteristic note and in oriental perfumes, for the addition of a spicy and heady note.
Natural availability :
Safranal was identified in several varieties of black and green tea (see Tea Absolute Colourless). It is one of the components of the fragrant principle of saffron and is present in Wormwood EO. Nevertheless, natural Safranal is not produced for the perfume industry.
Year of discovery :
First isolation and identification of Safranal was made in 1935.
Other comments :
Safranal has a smell quite similar to Ethyl Saranate, both used for their spicy notes of saffron and fruity notes. Nevertheless, the synthesis of Ethyl Safranate is less expensive and is preferred to Safranal.
Price Range :
€€€€
Stability :
Safranal double bonds gives a risk of polymerisation under the effect of oxydation.

Crédits photo: ScenTree SAS
- Molecular formula :
- C10H14O
- Molecular Weight :
- 150,22 g/mol
- Density :
- 0,966
- Flash Point :
- 86°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Pale yellow liquid
- Log P :
- Donnée indisponible,
- Boiling Point :
- 70°C (à 1 hPa)
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
The synthesis of Safranal has always been problematic. Whatever the production method, the yield of this molecule synthesis has never been high. One of the synthesis that offers a ''good '' yield starts from alpha-cyclocitral. This compound can undergo a bromination with phenyltrimethylammonium tribromide. The obtained intermediate is debrominated to give Safranal, with a yield that is still quite low. Using beta-cyclocitral for this synthesis offers only a complex mixture of molecules, of which Safranal cannot be isolated.
Synthesis precursor :
Safranal does not synthesize other compounds of olfactory interest.
Isomerism :
Safranal is a constitutional isomer of Thymol and L-Carvone among others, but has a very different structure and smell.
- EINECS number :
- 204-133-7
- FEMA number :
- 3389
- JECFA number :
- 977
- FLAVIS number :
- 05.104
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION
- Amendment :
- 49
- Comments :
- 2,6,6-Trimethylcyclohex-1,3-dienyl methanal has been found in natural extracts but only at trace levels.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,0022 % 0,00066 % 0,013 % 0,012 % 0,0032 % 0,0032 % 0,0032 % 0,0032 % 0,0073 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,025 % 0,025 % 0,0013 % 0,024 % 0,087 % 0,087 % 0,048 % 0,048 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 47
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,001 % 0,001 % 0,004 % 0,005 % 0,005 % 0,02 % 0,002 % 0,005 % 0,005 % 0,005 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.co to learn about our advertising opportunities.
