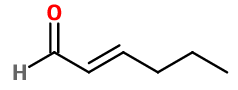
Trans-2-Hexenal
Naturelle - Synthétique
Fruity > Green Fruits > Boozy
Crédits photo: ScenTree SAS
Other names :
(E)-2-Hexenal ; Leaf aldehyde ; Trans-2-hexenyl aldehyde ; Trans-2-hexenal supra ; (E)-2-hexenyl aldehyde
Volatility :
Head
Uses in perfumery :
Trans-2-Hexenal is most often used in green notes and apple reconstitutions, to bring crunch to the fruit and this characteristic green note.
Natural availability :
Trans-2-Hexenal is present in several plant extracts, including the leaves of some trees and shrubs. These extracts include Geranium EO, Verbena EO or Petitgrain Grapefruit EO. It is also present in the fragrant principle of many fruits, always in small quantities. It is its synthetic version that remains the most widely used in perfumery.
Year of discovery :
Data not available.
Other comments :
Trans-2-Hexenal is very powerful and must be used diluted in compositions. It does not have the same smell, whether it is pure or diluted. It is its dilution that gives it its green and fruity facet, reminiscent of apple.
This molecule is to be distinguished from trans-2-Hexenol, its corresponding alcohol. Aldehyde is much more powerful, although both have a green, apple-like smell.
This molecule is to be distinguished from trans-2-Hexenol, its corresponding alcohol. Aldehyde is much more powerful, although both have a green, apple-like smell.
Price Range :
€€
Stability :
Aldehyde can form their diethylacetal in alcoholic stability, bringing not so much olfactive change, but less efficiency of the ingredient.

Crédits photo: ScenTree SAS
- Molecular formula :
- C6H10O
- Molecular Weight :
- 98,14 g/mol
- Density :
- 0,846
- Flash Point :
- 43°C
- Fusion Point :
- Donnée indisponible.
- Appearance :
- Colorless liquid
- Log P :
- 1,58
- Boiling Point :
- 146°C
- Detection Threshold :
- De l'ordre de quelques ppb, quelques dizaines de millionièmes de pourcents
Synthesis route :
The synthesis of trans-2-Hexenal can be achieved by reaction between two molar equivalents of butanal and one equivalent of ethyl vinyl ether, in the catalytic presence of boron trifluoride. This first synthesis step is followed by an acid hydrolysis of the obtained product, in the presence of concentrated sulphuric acid for example.
Biosynthetic ways of obtaining this molecule are being developed, improving its yield and reducing the rejections in the environment.
Synthesis precursor :
Trans-2-Hexenal, like all aldehydes, can be used to synthesize Schiff bases, by reaction with Methyl Anthranilate or Indole for example. These reactions often lead to the formation of a very powerful and coloured product.
Isomerism :
Trans-2-Hexenal is a diastereoisomer of cis-2-Hexenal, much less used in perfumery, but also with a very green and fruity note.
- EINECS number :
- 229-778-1
- FEMA number :
- 2560
- JECFA number :
- 1353
- FLAVIS number :
- 05.073
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Comments :
- trans-2-Hexenal has been found in natural extracts but only at trace levels.
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,0018 % 0,00055 % 0,011 % 0,01 % 0,0026 % 0,0026 % 0,0026 % 0,00087 % 0,006 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,021 % 0,021 % 0,00087 % 0,02 % 0,02 % 0,072 % 0,00087 % 0,00087 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 43
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,001 % 0,001 % 0,002 % 0,002 % 0,002 % 0,02 % 0,002 % 0,002 % 0,002 % 0,002 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

