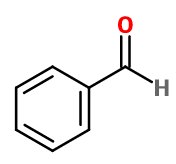
Benzaldehyde
Naturelle - Synthétique
Balsamic Ambery > Almondy > Vanillic
Crédits photo: ScenTree SAS
Other names :
Benzoic Aldehyde ; Almond artificial oil ; Benzanoaldehyde ; Benzarone ; Benzene carbaldehyde ; Benzene carbonal ; Benzene carboxaldehyde ; Benzene methylal ; Benzene carbinal ; Benzenecarbonal ; Benzenemehylal ; Benzoyl hydride ; Phenyl methanal ; Phenylformaldehyde ; Phenylketone ; Phenylmethanal
Volatility :
Head
Uses in perfumery :
Benzaldehyde is used in almond, fruit stone and nutty notes. Gives nuances to vanilla notes.
Natural availability :
Benzaldehyde can be found in almond, several fruits, Vanilla Bourbon Absolute (and other origins), Grandiflorum Jasmine Absolute and fruit stones. In its natural state, it is extracted from Bitter Almond EO, of which it is the major compound.
Year of discovery :
Discovered in 1863.
Other comments :
As it can be compared to Furfural and Salicylaldehyde, Benzaldehyde is the reference almond note in perfumery.
Price Range :
€
Stability :
Tends to oxydize easily into perbenzoic acid, which can react with itself to onvert it into Benzoic Acid.
Tends to color through time.
Tends to color through time.

Crédits photo: ScenTree SAS
- Molecular formula :
- C7H6O
- Molecular Weight :
- 106,12 g/mol
- Density :
- 1,045
- Flash Point :
- 64°C
- Fusion Point :
- -26°C
- Appearance :
- Colorless viscous liquid
- Log P :
- 1,5
- Boiling Point :
- 179°C
- Detection Threshold :
- Varie selon les personnes entre 100 ppb et 4,6 ppm (0,00046%), lorsque son seuil de reconnaissance varie entre 330 ppb et 4,1 ppm,
Synthesis route :
Benzaldehyde is synthesized by hydrolysis of benzyl chloride, by an acidic route in the presence of iron chloride, or by an alkaline route in the presence of sodium carbonate. A toluene oxidation also allows to obtain both Benzoic Acid and Benzaldehyde.
Synthesis precursor :
Benzaldehyde is the precursor to the synthesis of many compounds used in perfumery. For example, its hydrogenation synthesizes benzoic alcohol. It condenses with other aldehydes to create many other compounds, replacing the aldehyde function with an alcene function. Finally, a Perkin reaction with several acids allows to obtain various compounds, such as Cinnamic Acid by reaction with acetic anhydride. Finally, benzaldehyde forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Isomerism :
Benzaldehyde does not have any isomer used in perfumery.
- EINECS number :
- 202-860-4
- FEMA number :
- 2127
- JECFA number :
- 22
- FLAVIS number :
- 05.013
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Comments :
- This ingredient is part of the Schiff base (Benzaldehyde methyl anthranilate (or Amandolene) - N°CAS : 39129-16-3) and induces the application of IFRA regulations for 44,4% of the Schiff base usage. Please also refer to the IFRA Annex II for more information
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,045 % 0,014 % 0,27 % 0,25 % 0,064 % 0,064 % 0,064 % 0,021 % 0,15 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,52 % 0,52 % 0,021 % 0,49 % 0,49 % 1,8 % 0,021 % 0,021 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- SENSITIZATION
- Amendment :
- 47
- Comments :
- This ingredient is part of the Schiff base (Benzaldehyde methyl anthranilate (or Amandolene) - N°CAS : 39129-16-3) and induces the application of IFRA regulations for 44,4% of the Schiff base usage. Please also refer to the IFRA Annex II for more information
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,02 % 0,02 % 0,09 % 0,27 % 0,14 % 0,43 % 0,05 % 0,6 % 3 % 2,5 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


