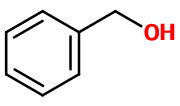
Benzyl Alcohol
Naturelle - Synthétique
Floral > White Flowers > Benzyl > Plastic
Crédits photo: ScenTree SAS
Other names :
Alpha-hydroxytoluène ; Benzencarbinol ; Benzene carbinol ; Benzene methanol ; Benzoyl alcohol ; Benzylicum ; (hydroxymethyl)benzene ; Phenyl methanol ; Phenyl carbinol ; Phenyl carbinolum ; Alpha-toluenol ; Ulfesia
Volatility :
Heart/Base
Uses in perfumery :
Benzyl Alcohol is used in majority as a solvent for some ingredients, even if it is rarely the case. It can also be used is some white flowers accords, to bring volatility.
Natural availability :
Benzyl Alcohol is found in low quantity in many natural ingredients. It is found for example in Clove Leaf EO, Damask Rose Absolute and Narcissus Absolute.
Year of discovery :
Data not available.
Other comments :
In perfumery, using a benzylic molecule brings a fruity white flower note as jasmine. A long homologous molecule of Benzyl Alcohol is Phenyl Ethyl Alcohol, bringing a rosy note. By lengthening even more the chain, a dihydrocinnamic body brings more heavy floral balsamic note.
Benzyl Alcohol is part of the 26 allergens used in perfumery.
Benzyl Alcohol is part of the 26 allergens used in perfumery.
Price Range :
€
Stability :
Aromatic compounds are chromophorous. This means that they may colour through time or in an alkaline medium.

Crédits photo: ScenTree SAS
- Molecular formula :
- C7H8O
- Molecular Weight :
- 108,14 g/mol
- Density :
- 1,04
- Flash Point :
- 93°C
- Fusion Point :
- - 15°C
- Appearance :
- Colorless liquid
- Log P :
- 1,1
- Boiling Point :
- 205°C
- Detection Threshold :
- 1,2 et 1000 ppb (0,0001%) selon les personnes
Synthesis route :
Two synthetic routes can be used to obtain Benzyl Alcohol. The first one is a hydrolysis of benzyl chloride, by heating this molecule with alkaline and earthy-alkaline hydroxydes and carbonates. Up to 10% Dibenzyl Ether is also resulting from this reaction. A second route is an oxydation of toluene into benzyl hydroperoxyde. Subsequent hydrolysis synthesizes Benzyl Alcohol and Benzaldehyde. A purification can then isolate Benzyl Alcohol.
Synthesis precursor :
Benzyl Alcohol can be used to synthesize Benzaldehyde, by adding nitric acid to oxydize it. This oxydation can also be carried out with copper-magnesium oxide pumice. Numerous esterifications can be carried out using Benzyl Alcohol, to obtain compounds as Benzyl acetate, Benzyl Salicylate... Eventually, heating this molecule with strong acids or bases may form Dibenzyl Oxide.
Isomerism :
Benzyl Alcohol is an isomer of para-Cresol. These two molecules, although close structurally speaking, do not have the same smell at all, much more animalic on para-Cresol part.
- EINECS number :
- 202-859-9
- FEMA number :
- 2137
- JECFA number :
- 25
- FLAVIS number :
- 02.010
- Allergens :
- Benzyl Alcohol may provoke an allergic reaction on skin contact (redness, heat, scraching, prickling) for some people.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,45 % 0,14 % 0,34 % 2,5 % 0,64 % 0,17 % 0,34 % 0,057 % 1,5 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 0,68 % 0,68 % 0,057 % 2,2 % 2,2 % 8,5 % 0,057 % 0,057 % No Restriction - Restriction type :
- RESTRICTION QRA
- Cause of restriction :
- Amendment :
- 42
- Quantitative usage limits :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5 Cat.6 Cat.7 Cat.8 Cat.9 Cat.10 Cat.11 0,2 % 0,2 % 0,9 % 2,7 % 1,4 % 4,3 % 0,4 % 2 % 5 % 2,5 % Not Restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


