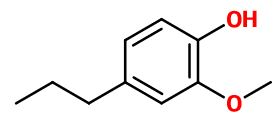
DihydroEugenol
Synthétique
Spicy > Warm Spices > Eugenolic > Metallic
Crédits photo: ScenTree SAS
Other names :
2-methoxy-4-propylphenol ; 4-propylguaiacol ; Ortho-5-propylhydroxyanisole ; Ortho-4-propylmethoxyphenol ; Cerulignol ; Coerulignol ; Guaiacylpropane ; Cerulignol ; Cerulignol ; Dihydro eugenol ; Guaiacyl propane ; 4-hydroxy-3-methoxypropyl benzene ; (4-hydroxy-3-methoxyphenyl) propane ; 4-propyl guaiacol ; Para-propyl guaiacol ; 5-propyl ortho-hydroxyanisole ; 4- propyl-2-methoxyphenol
Volatility :
Head/Heart
Uses in perfumery :
DihydroEugenol is used to replace Eugenol is some accords, as it is not a regulated ingredient, for the same reasons as Eugenol, but mostly when the aim is to bring more facets to fresh spices such as black pepper, cardamom etc.
Natural availability :
DihydroEugenol has been identified in diverse consumer goods such as some whiskeys and cheeses. Nevertheless, it is not extracted from any plant, to get it on a natual way.
Year of discovery :
Data not available.
Other comments :
Compared to Eugenol, Dihydroeugenol has an even more metallic smell, closing it to Clove Bud EO.
Price Range :
€€
Stability :
Becomes red under during light exposure and in an alcaline base. This is limiting the use of this raw material in alcaline bases such as shower gel or shampoo bases among others.

Crédits photo: ScenTree SAS
- Molecular formula :
- C10H14O2
- Molecular Weight :
- 166,22 g/mol
- Density :
- 1,038
- Flash Point :
- 113°C
- Fusion Point :
- 17°C
- Appearance :
- Pale yellow liquid
- Log P :
- Donnée indisponible,
- Boiling Point :
- 240°C
- Detection Threshold :
- Donnée indisponible.
Synthesis route :
DihydroEugenol can be synthetised starting with Isoeugenol or Eugenol, using a catalytical hydrogenation reaction. This reaction consists in putting the molecule under a large pressure of hydrogen, in the presence of a metallic catalyst on carbon, such as platinium or palladium. The insaturation present in Isoeugenol or Eugenol is transformed into a simple bond in the final product.
Synthesis precursor :
DihydroEugenol is not a precursor to the synthesis of any other molecule used in perfumery.
Isomerism :
DihydroEugenol is a constitutional isomer of Viridine®, even if it doesn't have the same smell at all.
- EINECS number :
- 220-499-0
- FEMA number :
- 3598
- JECFA number :
- 717
- FLAVIS number :
- 04.049
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is restricted by IFRA
- Restriction type :
- RESTRICTION
- Cause of restriction :
- DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
- Amendment :
- 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A Cat.5B Cat.5C Cat.5D Cat.6 0,13 % 0,039 % 0,78 % 0,73 % 0,19 % 0,19 % 0,19 % 0,062 % 0,43 % Cat.7A Cat.7B Cat.8 Cat.9 Cat.10A Cat.10B Cat.11A Cat.11B Cat.12 1,5 % 1,5 % 0,062 % 1,4 % 1,4 % 5,1 % 0,062 % 0,062 % No Restriction - Annexe I :
- Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Birch tar oil, rectified | Betula spp. | 8001-88-5 | 0,9 |
| Cade oil, rectified | Juniperus oxycedrus L. | 8013-10-3 | 0,5 |
This ingredient is not restricted for the 48th amendment
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.


