Terpinyl acetate
Naturelle - Synthétique
Citrus > Zesty > Bergamot > Agrestic
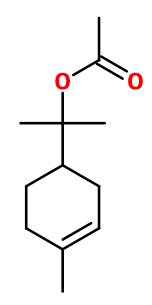
Crédits photo: ScenTree SAS
Other names :
2-(4-methyl-1-cyclohex-3-enyl)propan-2-yl acetate ; Terpenyl acetate ; Terpinyl ethanoate ; Terpinyl ethanoate ; Lindenyl acetate ; Para-menthene-8-yl acetate
Volatility :
Head
Uses in perfumery :
Terpinyl acetate is used to reproduce bergamot at a lower cost, as its smell is so close to linalyl acetate, but more associated with cheap perfumes.
Natural availability :
Terpinyl acetate is present in a large quantity in certain varieties of Cardamom EO, Laurel EO, Clary Sage EO and Red Thyme EO, from which it can be extracted in its natural state.
Year of discovery :
Data not available.
Other comments :
In terms of smell, Terpinyl acetate is to be compared with Linalyl acetate and Menthanyl acetate. It brings an additional sweet facet.
Price Range :
€€
Stability :
acetates may form acetic acid through time

Crédits photo: ScenTree SAS
- Molecular formula :
- C12H20O2
- Molecular Weight :
- 196,29 g/mol
- Density :
- 0,96
- Flash Point :
- 101°C
- Fusion Point :
- -20°C
- Appearance :
- Colorless liquid
- Log P :
- 4,4
- Boiling Point :
- 232°C
- Detection Threshold :
- 2,5 ppm (0,00025%)
Synthesis route :
The synthesis of Terpenyl acetate can be made by esterification of alpha-Terpineol in the presence of acetic acid or acetic anhydride, but also from alpha-Pinene which is easily extractable naturally, in two steps. Plunging alpha-Pinene in an aqueous acidic medium, converts it to 1,8-terpin with a 90% yield. When this molecule is placed in an acid medium, alpha-Terpineol and its derivatives are formed, including Terpenyl acetate. Then, it is removed from the mixture by fractional distillation.
Synthesis precursor :
Terpenyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Isomerism :
Terpenyl acetate has an asymmetric carbon. The two enantiomers are not separated to be used in perfumery.
Geranyl acetate, Neryl acetate, Linalyl acetate and Isobornyl acetate are isomers of Terpenyl acetate. Only Linalyl acetate has a smell of Bergamot EO, although more zesty. Geranyl acetate and Neryl acetate smell like pear and rose, while Isobornyl acetate is close to pine.
- EINECS number :
- 201-265-7
- FEMA number :
- 3047
- JECFA number :
- 368
- FLAVIS number :
- 09.015
- Allergens :
- This ingredient does not contain any allergen.
- IFRA :
- This ingredient is not restricted
To learn more about IFRA's standards : https://ifrafragrance.org/safe-use/library
ScenTree is solely responsible for the information provided here.

